Efficacy data
PrTAVNEOS® achieved superior sustained remission compared to prednisone-based group at 52 weeks (primary endpoint, ITT population)1*

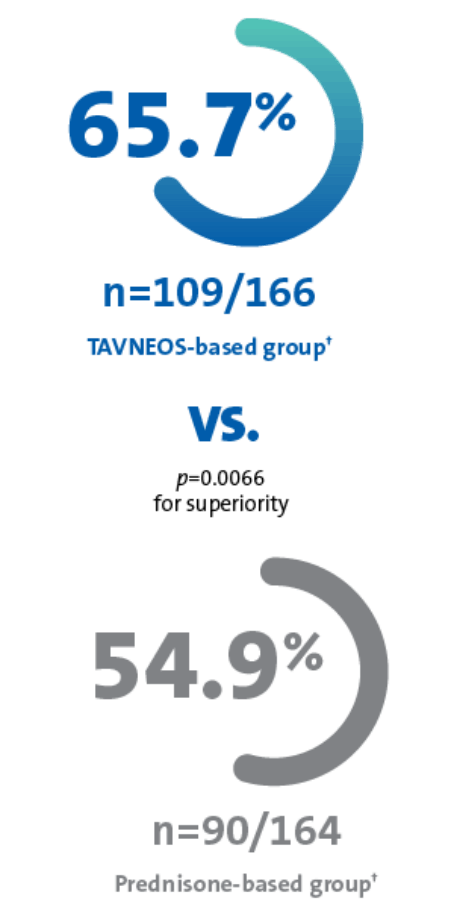
At 26 weeks, TAVNEOS demonstrated non-inferiority in the proportion of subjects achieving remission vs. prednisone-based group: 72.3% vs. 70.1%, respectively (primary endpoint; p<0.0001 for non-inferiority)1*
ADVOCATE trial study design1
In the phase 3 ADVOCATE trial, patients with newly diagnosed or relapsing active ANCA-associated vasculitis received either:*†
TAVNEOS-based group
(n=166)
TAVNEOS and
prednisone-matching placebo
Prednisone-based group
(n=164)
Prednisone and TAVNEOS-matching placebo

One of the following standard immunosuppressive regimens:
- IV cyclophosphamide followed by oral azathioprine or mycophenolate mofetil
- Oral cyclophosphamide followed by oral azathioprine or mycophenolate mofetil
- Rituximab
Patients were stratified at randomization based on three factors:
- Receiving either IV rituximab, IV cyclophosphamide, or oral cyclophosphamide
- Having proteinase 3 (PR3) or myeloperoxidase (MPO) ANCAs
- Newly diagnosed or relapsing disease
Primary endpoints
- Remission at week 26: Birmingham Vasculitis Activity Score (BVAS)=0 and no glucocorticoid use for AAV within 4 weeks before week 26
- Sustained remission at week 52: remission at week 26 and week 52, without relapse through week 52, and no glucocorticoid use for AAV within 4 weeks before week 52
*
The ADVOCATE trial was a phase 3, randomised, double-blind, double-dummy, active-controlled clinical trial, assessing the efficacy, safety profile, and tolerability of avacopan in subjects with newly diagnosed or relapsing active ANCA-associated vasculitis when administered against a standard background cyclophosphamide or rituximab regimen. The trial treatment period was 52 weeks with an 8-week follow-up period.
†
Subjects received either 30 mg TAVNEOS twice daily for 52 weeks plus prednisone-matching placebo tapering regimen over 20 weeks in the TAVNEOS-based group (n=166) or TAVNEOS-matching placebo twice daily for 52 weeks plus prednisone (tapered from 60 mg/day to 0 over 20 weeks) in the prednisone-based group (n=164). In addition, all patients received standard immunosuppressive regimens of either:
- IV cyclophosphamide for 13 weeks (15 mg/kg up to 1.2 g every 2 to 3 weeks) followed by oral azathioprine (1 mg/kg daily with titration up to 2 mg/kg daily; mycophenolate mofetil 2 g daily was allowed in place of azathioprine) starting on week 15; or
- Oral cyclophosphamide for 14 weeks (2 mg/kg daily) followed by either oral azathioprine or mycophenolate mofetil (same dosing regimen as IV cyclophosphamide) starting at week 15; or
- Rituximab at the dose of 375 mg/m² for 4 weekly IV doses.
Reference:
1. TAVNEOS Product Monograph. Otsuka Canada Pharmaceutical Inc.
